So, you’re thinking about Exporting Indian Herbal and Natural Cosmetics to Germany? Great choice! Germany is one of the biggest beauty markets in Europe, and consumers there love herbal, natural, and clean-label products. But here’s the catch: Germany (like the rest of the EU) has some of the strictest cosmetic regulations in the world.
Don’t let that scare you. With the right prep, you can absolutely get your herbal oils, ayurvedic creams, or natural serums onto German shelves. In this guide, we’ll break it all down in plain language from Regulation (EC) 1223/2009 to CPNP notifications, from claims substantiation to French language labelling (yep, that matters too if you branch out into France).
Why Exporting Indian Herbal and Natural Cosmetics to Germany Is Worth It
Germany isn’t just another European market — it’s home to established drugstore chains, pharmacies, and eco-retailers that prioritize transparency, ingredient safety, and product authenticity.
Consumers expect natural products to be backed by clear documentation, and regulatory frameworks reinforce those expectations through strict compliance standards.
Regulation (EC) 1223/2009 and Its Role in Exporting Indian Herbal and Natural Cosmetics to Germany
Think of Regulation (EC) 1223/2009 as the EU cosmetics bible. It tells you what you need in terms of:
- Safety (via a Cosmetic Product Safety Report (CPSR))
- Documentation (the Product Information File (PIF))
- Manufacturing standards (GMP ISO 22716)
- Ingredient rules (prohibited/restricted substances and CMR substances)
- Who’s legally responsible (your EU Responsible Person (RP))
- Notification before market (CPNP portal)
If you get these right, you’re on track.

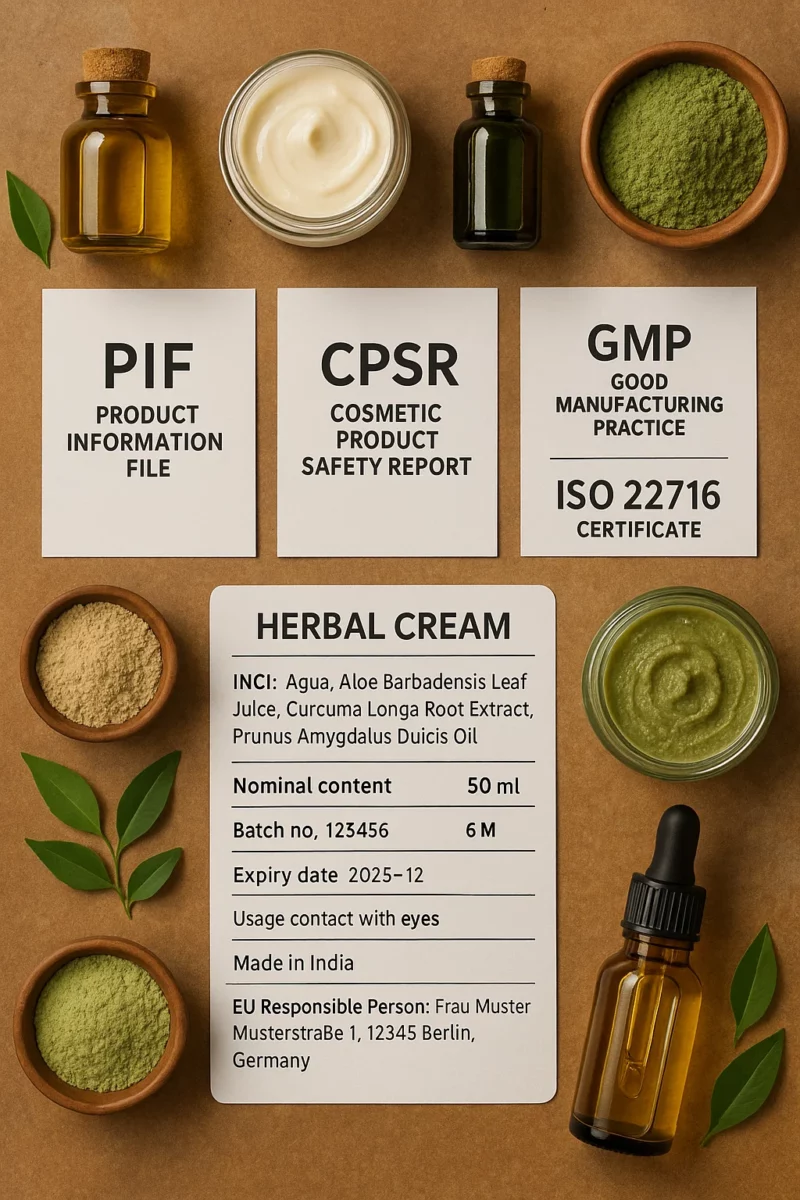
Product Information File (PIF): The Core of Exporting Indian Herbal and Natural Cosmetics to Germany
When Exporting Indian Herbal and Natural Cosmetics to Germany, you’ll need a PIF for each product. Think of the PIF as your compliance dossier. Authorities can ask for it at any time, and you have to keep it for 10 years after the last batch hits the market.
What goes inside?
- A CPSR (the safety report – more on this next)
- Proof that you follow Good Manufacturing Practices (GMP ISO 22716)
- Ingredient safety data (checking for prohibited substances, restricted substances, and CMR substances)
- Any relevant SCCs opinions you relied on
- Label copies (with INCI names, nominal content, batch number, PAO, expiry date, country of origin labelling)
- Claims substantiation documents (basically, proof your product does what you say it does, and that you’re not making medicinal claims)
Cosmetic Product Safety Report (CPSR) for Exporting Indian Herbal and Natural Cosmetics to Germany
The Cosmetic Product Safety Report (CPSR) is mandatory under Regulation (EC) 1223/2009. This isn’t something you DIY, you’ll need a qualified safety assessor (toxicologist or similar) to sign off.
For herbal and natural cosmetics, expect extra scrutiny. Botanicals can come with impurities like heavy metals, pesticide residues, or even traces of CMR substances. The CPSR must:
- List all ingredients by INCI names
- Review toxicology for each one
- Double-check for prohibited/restricted substances
- Factor in SCCS guidance where relevant
- Conclude that your product is safe to use
Good Manufacturing Practices (GMP ISO 22716) in Exporting Indian Herbal and Natural Cosmetics to Germany
Even if you’re producing in a small unit in India, your processes need to align with Good Manufacturing Practices (GMP ISO 22716). This standard covers hygiene, equipment, raw material controls, and batch records. Mention your GMP compliance in the PIF, and keep certificates handy if you’ve been audited.
Ingredients – The Tricky Part
Botanical Restrictions
Some plant-based ingredients contain naturally occurring restricted or prohibited substances, such as certain coumarins.
CMR Substance Risk
If there’s potential exposure to CMR substances, scientific data must demonstrate that the extract is safe for use.
Check CosIng Database
Verify INCI names, usage conditions, and restrictions using the CosIng database before product formulation or export.
Monitor SCCS Opinions
Safety statuses evolve — keep track of SCCS scientific opinions as they regularly update ingredient assessments.
Appoint an EU Responsible Person (RP)
You cannot sell cosmetics in Germany without a designated EU Responsible Person (RP). This is a company or individual in the EU who ensures your compliance.
The RP’s name and physical address must appear on your product label (the famous RP address on label rule). If you’re based in India, you’ll usually hire an EU RP service provider or work with your importer.
Notify your products in the CPNP
Before your herbal face cream can sit on a shelf in Berlin, it must be uploaded into the Cosmetic Products Notification Portal (CPNP). The RP normally handles this, but you’ll need to supply:
- Product details
- PIF reference
- Labels with INCI list, nominal content, batch number, PAO, expiry date, usage precautions, country of origin
Once notified, poison centers and regulators can access the info if needed.
Labels that Tick all the Boxes
Your German customers will flip the product around and check the label, and so will regulators. Here’s what you need to include:
- INCI names (not local/common names)
- Nominal content (ml/g)
- Batch number
- PAO symbol or expiry date
- Country of origin labelling (helpful for customs and transparency)
- Usage precautions (in German!)
- RP name and postal address
If you’re expanding into France later, remember: French language labelling (cosmetics) is mandatory there, thanks to the Code de la santé publique and DGCCRF oversight.
Keep Claims Clean (and Legal)
Want to say your ayurvedic cream “treats acne”? Don’t. That’s a medicinal claim and regulators will block you.
Instead, use cosmetic-friendly wording and back it up with claims substantiation. For example, “helps reduce the appearance of blemishes” is acceptable if you have test data.
Be Ready for Vigilance Reporting
Once your products are on the market, your job isn’t over. The RP must monitor for customer complaints and adverse effects.
- Minor reactions? Log them.
- Serious Undesirable Effects (SUE)? These must be reported to authorities right away (SUE reporting).
This whole system is called vigilance reporting, and it’s part of keeping your PIF up to date.
Quick Recap Checklist for Exporting Indian Herbal and Natural Cosmetics to Germany
Final Thoughts
Exporting Indian Herbal and Natural Cosmetics to Germany isn’t just about having a beautiful product, it’s about showing regulators that it’s safe, compliant, and traceable.
If you:
- Invest in a strong PIF and CPSR,
- Follow GMP ISO 22716,
- Work with a trustworthy EU RP,
- Nail your CPNP notification, and
- Keep your labels, claims, and vigilance system clean…
…then you’re ready to tap into one of the most promising natural beauty markets in the world.
FAQs
The first step is to check compliance with Regulation (EC) No.1223/2009, which governs all cosmetics in the EU. You’ll need a Product Information File (PIF), a Cosmetic Product Safety Report (CPSR), and an appointed EU Responsible Person (RP) before placing products on the German market.
Yes. GMP ISO 22716 is the recognized manufacturing standard in the EU. Following GMP ensures consistent quality, hygiene, and traceability, which is mandatory when exporting Indian Herbal and Natural Cosmetics to Germany.
A PIF must include the CPSR, product description, manufacturing details with GMP compliance, ingredient safety data, labels (with INCI names, nominal content, batch number, PAO or expiry date, usage precautions, and country of origin labelling), and claims substantiation.
The Responsible Person (RP) is legally accountable for compliance in the EU. Their address must be printed on the label, and they must handle CPNP notification, vigilance reporting, and Serious Undesirable Effects (SUE) reporting.
Yes. Every cosmetic product must be uploaded to the Cosmetic Products Notification Portal (CPNP) before being sold in Germany. Without this, your product cannot legally enter the EU market.